Diffusion across a semipermeable membrane lab – In the realm of scientific inquiry, diffusion across a semipermeable membrane stands as a fundamental concept, elucidating the movement of molecules across selective barriers. This laboratory investigation delves into the intricacies of this phenomenon, offering a hands-on approach to understanding its mechanisms and implications.
Through meticulously designed experiments, students will explore the principles of diffusion, osmosis, and the role of semipermeable membranes in biological systems. This immersive experience fosters a deeper comprehension of the principles that govern the movement of substances across cellular boundaries, shaping our understanding of cellular processes and physiological phenomena.
Introduction
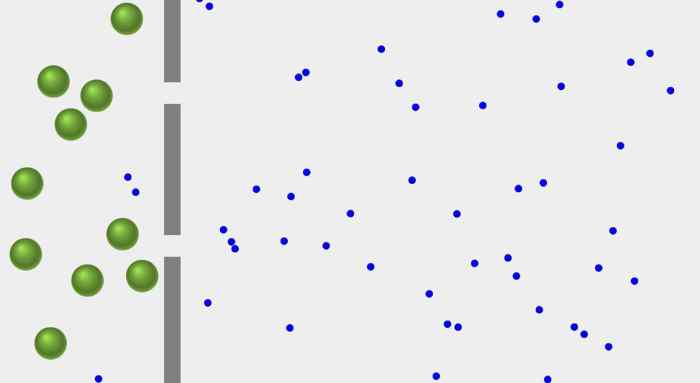
Diffusion is the net movement of molecules from an area of high concentration to an area of low concentration. Osmosis is a specific type of diffusion that involves the movement of water across a semipermeable membrane. A semipermeable membrane is a membrane that allows some molecules to pass through but not others.
The purpose of this lab is to investigate the factors that affect the rate of diffusion across a semipermeable membrane.
Materials
- 100 mL of 1 M NaCl solution
- 100 mL of 1 M sucrose solution
- 200 mL of distilled water
- 2 dialysis bags
- 2 beakers
- 1 graduated cylinder
- 1 balance
Procedure
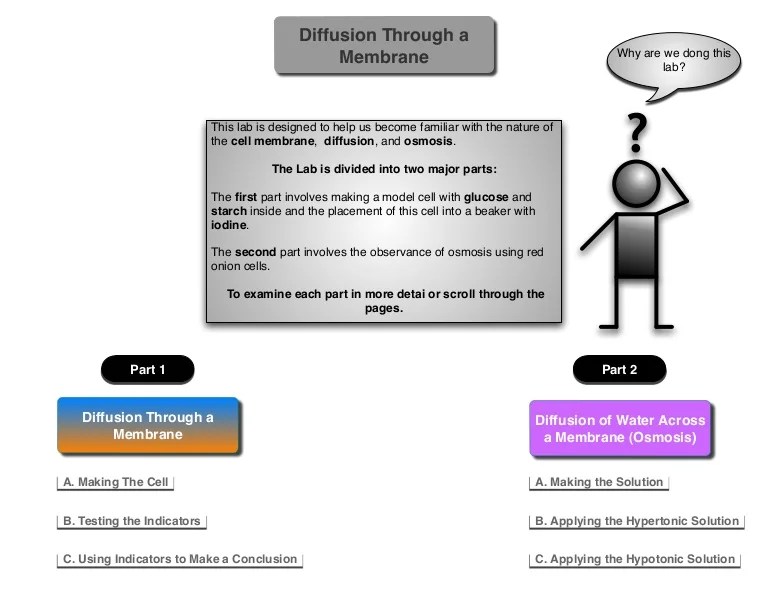
- Fill one dialysis bag with 100 mL of 1 M NaCl solution and the other dialysis bag with 100 mL of 1 M sucrose solution.
- Tie off the ends of the dialysis bags and place them in separate beakers.
- Fill one beaker with 100 mL of distilled water and the other beaker with 100 mL of 1 M NaCl solution.
- Place the dialysis bags in the beakers and stir the solutions gently.
- Record the mass of each dialysis bag at 10-minute intervals for 30 minutes.
Data Analysis

- Calculate the rate of diffusion for each dialysis bag by dividing the change in mass by the time interval.
- Create a table or graph to organize the data.
- Discuss the factors that affect the rate of diffusion.
FAQ Explained: Diffusion Across A Semipermeable Membrane Lab
What is the purpose of a semipermeable membrane?
A semipermeable membrane selectively allows certain molecules to pass through while blocking others, creating a concentration gradient that drives diffusion.
How is the rate of diffusion measured in this lab?
The rate of diffusion is calculated by measuring the change in concentration of a substance over time, using techniques such as spectrophotometry or conductivity.
What factors affect the rate of diffusion?
Factors such as temperature, concentration gradient, surface area, and membrane thickness influence the rate of diffusion across a semipermeable membrane.