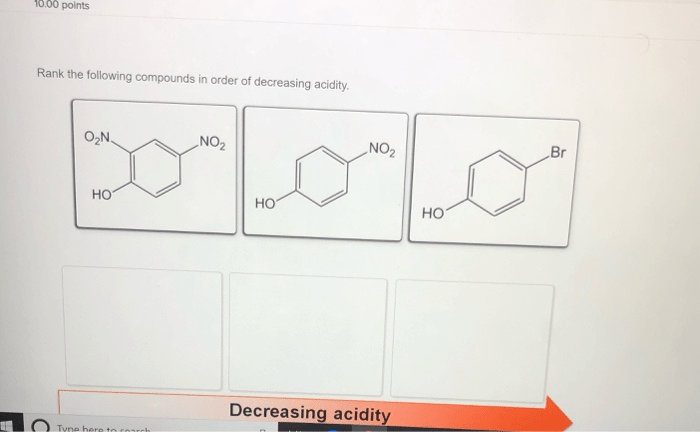
Rank the following in order of decreasing acidity – Delving into the realm of acidity, this guide embarks on a journey to explore the concept of acidity, its measurement, and the factors that influence it. By ranking various acids in order of decreasing acidity, we gain a deeper understanding of their chemical properties and practical applications.
Acidity, a fundamental property of acids, is quantified using the pH scale, which ranges from 0 to 14. Acids, characterized by their ability to donate protons (H+ ions), exhibit pH values below 7. This guide delves into the factors that affect acidity, including the strength of the acid, its concentration, and the presence of other ions in solution.
Acids: Rank The Following In Order Of Decreasing Acidity
Acids are chemical substances that can donate a proton (H+ ion) to another substance. They are typically sour, corrosive to skin, and react with bases to form salts. Examples of acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3).
Acids have several characteristic properties. They turn blue litmus paper red, react with metals to produce hydrogen gas, and react with carbonates to produce carbon dioxide gas. Acids also have a sour taste and can cause burns on the skin.
Acidity
Acidity is a measure of the concentration of hydrogen ions (H+) in a solution. The pH scale is used to measure acidity, with a pH of 7 being neutral, a pH below 7 being acidic, and a pH above 7 being basic.
Several factors can affect the acidity of a solution. These factors include the concentration of the acid, the temperature of the solution, and the presence of other ions in the solution.
Ranking Acids by Acidity
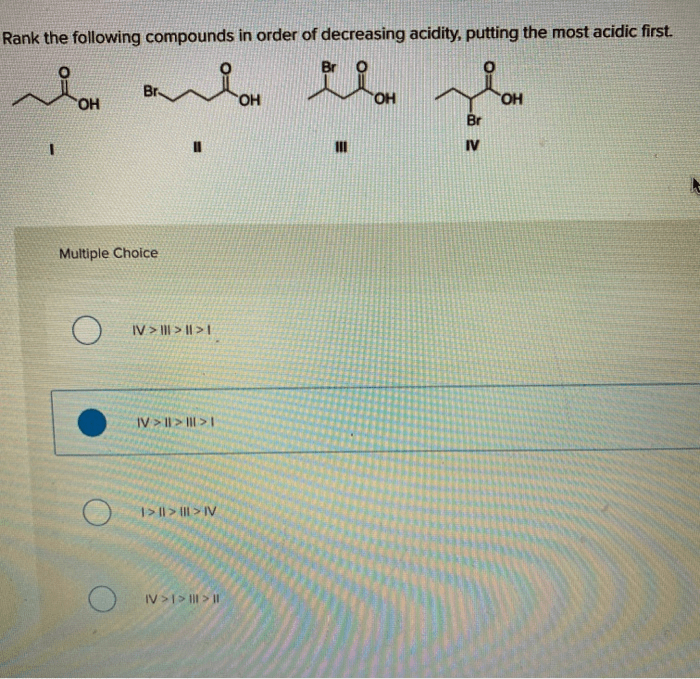
| Acid | Formula | pH |
|---|---|---|
| Hydrochloric acid | HCl | 0 |
| Sulfuric acid | H2SO4 | -2 |
| Nitric acid | HNO3 | -1 |
| Acetic acid | CH3COOH | 2.4 |
| Carbonic acid | H2CO3 | 6.3 |
The table above shows a list of acids and their corresponding pH values. The acids are ranked in order of decreasing acidity, with hydrochloric acid being the most acidic and carbonic acid being the least acidic.
Applications of Acidity
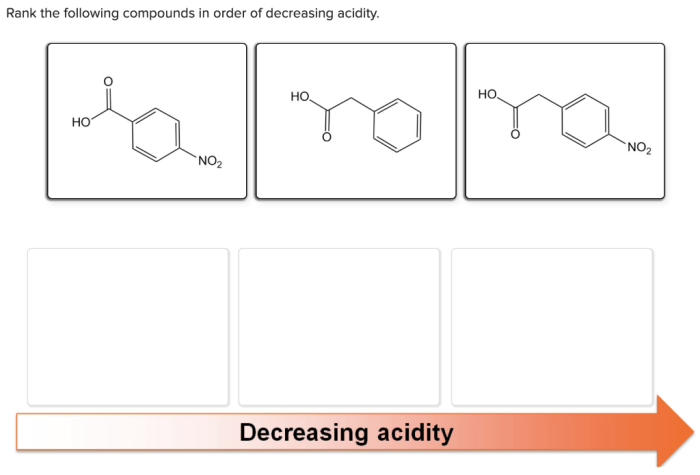
Acids have a wide range of applications in various fields. In the chemical industry, acids are used to produce fertilizers, plastics, and other chemicals. In the food industry, acids are used to preserve food and to add flavor to food.
In the medical industry, acids are used to treat a variety of conditions, including heartburn, indigestion, and diarrhea.
FAQ Explained
What is the definition of an acid?
An acid is a substance that donates protons (H+ ions) when dissolved in water, resulting in a pH value below 7.
How is acidity measured?
Acidity is measured using the pH scale, which ranges from 0 to 14. Acids have pH values below 7.
What factors affect the acidity of an acid?
The acidity of an acid is influenced by its strength, concentration, and the presence of other ions in solution.