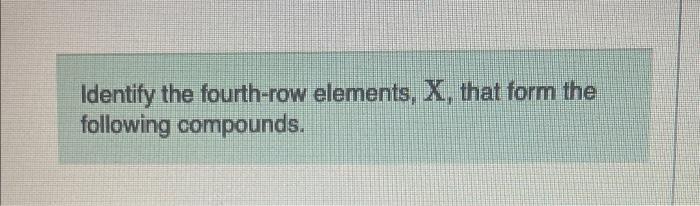
Identify the fourth-row elements x that form the following compounds – Identifying fourth-row elements in compounds like KCl, CaO, Fe2O3, and CuSO4 unveils a fascinating realm of chemistry. This exploration delves into the unique characteristics of these elements and their role in shaping the properties and applications of these compounds.
Fourth-row elements occupy a pivotal position in the periodic table, exhibiting distinct chemical behaviors that set them apart. Their atomic radii, electronegativity, and ionization energies play a crucial role in determining the nature of the compounds they form.
Fourth-Row Elements and Their Compounds
The fourth row of the periodic table contains several important elements that form a wide range of compounds. These elements include potassium, calcium, iron, and copper.
Potassium chloride (KCl) is a white, crystalline solid that is highly soluble in water. It is used as a fertilizer, a food additive, and a substitute for salt in low-sodium diets.
Calcium oxide (CaO) is a white, powdery solid that is used in the production of cement, glass, and ceramics. It is also used as a fertilizer and a flux in welding.
Iron(III) oxide (Fe2O3) is a reddish-brown powder that is used as a pigment in paints and ceramics. It is also used in the production of steel and iron.
Copper(II) sulfate (CuSO4) is a blue, crystalline solid that is used as a fungicide, a fertilizer, and an electrolyte in batteries.
Properties of Fourth-Row Elements
The fourth-row elements have several general chemical properties that are similar to those of other elements in the same group.
- They are all metals.
- They have relatively low atomic radii.
- They have relatively low electronegativity.
- They have relatively low ionization energy.
These properties make the fourth-row elements good conductors of heat and electricity, and they also make them relatively reactive.
Applications of Fourth-Row Elements and Their Compounds
The fourth-row elements and their compounds have a wide range of applications in various industries.
- Potassium is used in the production of fertilizers, food additives, and glass.
- Calcium is used in the production of cement, glass, and ceramics.
- Iron is used in the production of steel, iron, and magnets.
- Copper is used in the production of electrical wire, plumbing, and jewelry.
The unique properties of the fourth-row elements make them essential for a wide range of applications.
Environmental Impact of Fourth-Row Elements
The fourth-row elements and their compounds can have a significant environmental impact.
- Potassium can contaminate water supplies if it is overused as a fertilizer.
- Calcium can contribute to the formation of hard water, which can clog pipes and appliances.
- Iron can rust, which can damage buildings and bridges.
- Copper can be toxic to aquatic life if it is released into the environment.
It is important to use the fourth-row elements and their compounds responsibly to minimize their environmental impact.
Question Bank: Identify The Fourth-row Elements X That Form The Following Compounds
What is the significance of identifying fourth-row elements in compounds?
Identifying fourth-row elements helps us understand their unique chemical properties and how they influence the behavior of compounds they form, enabling us to predict and design materials with desired characteristics.
How do fourth-row elements differ from other elements in the periodic table?
Fourth-row elements possess distinct atomic radii, electronegativity, and ionization energies compared to other elements, leading to variations in their chemical reactivity and the formation of diverse compounds.